The stevia market is diversifying rapidly - which next-generation derivatives should formulators watch?
Reb M and enzyme-modified glucosylated stevia currently show the fastest growth (45% CAGR), driven by clean-label beverage reformulations. Our production data reveals:

Emerging derivatives demand different sourcing strategies compared to traditional Reb A.## How to Identify Fake or Adulterated Stevia Products?
Counterfeit stevia costs the industry $180M annually - what red flags distinguish authentic product?
Genuine stevia exhibits:
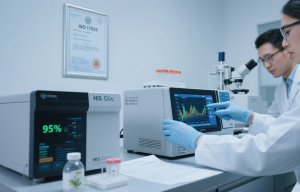
- Purity over 95% Reb A/M/D
- Bitter aftertaste (absence suggests maltodextrin dilution)
- Solubility under 70°C (instant dissolving indicates carriers)
Common adulterants and detection methods:
| Adulterant | Purpose | Detection Test | Cost Impact |
|---|---|---|---|
| Maltodextrin | Bulking agent | HPLC purity analysis | 22-35% cheaper |
| Erythritol | Mask bitterness | Sugar alcohol test | 40% higher margin |
| Artificial sweeteners | Enhance sweetness | MS/GC testing | Dangerous for exports |
Our ISO 17025 accredited lab provides authenticity certificates [4][5].## Does Organic Stevia Offer Better Profit Margins?
Organic premiums reach 80% - but do real returns justify certification costs?
Verified organic stevia delivers 35-50% higher margins though requires 18-month transition planning. Key considerations:

Breakdown of organic economics:
-
Input Costs
- Organic seedlings cost 2.5× more
- Hand weeding adds $500/acre labor
-
Yield Impact
- 25-30% lower leaf yield
- But 20% higher glycoside content [3]
-
Market Access
- EU buyers pay 60-80% premium
- US retail labels command 50% markup
Our organic program includes contract farming with traceability back to specific plots [2][5].## How Does Stevia Compliance Differ Across Countries?
Regulatory fragmentation creates trade headaches - what are the key market barriers?
Major market requirements comparison:
| Market | Sweetener Approval | Labeling Rules | Testing Requirements |
|---|---|---|---|
| USA | GRAS Notice | "Stevia Extract" permitted | Solvent residue analysis |
| EU | Novel Food Authorization | "Steviol Glycosides" required | Heavy metals below 0.1ppm |
| Japan | Food Additive Listing | JAS Seal mandatory | Radiation certificate |
| Middle East | Halal Certification | No Alcohol Processing | GMO-free verification |
Our regulatory team maintains current dossiers in 38 countries, including:
- FDA GRAS No. GRN000253
- EFSA Novel Food 2011/13/EU
- China NHFPC 2012 Safety Approval [4]